All Recalled Eye Drops 2025 Schedule. The drops, used to relieve itchy, dry eyes, were also recalled due to a “lack of assurance of sterility,” according to the fda. If you’re a frequent eye drop user, now’s the time to check your medicine cabinet:
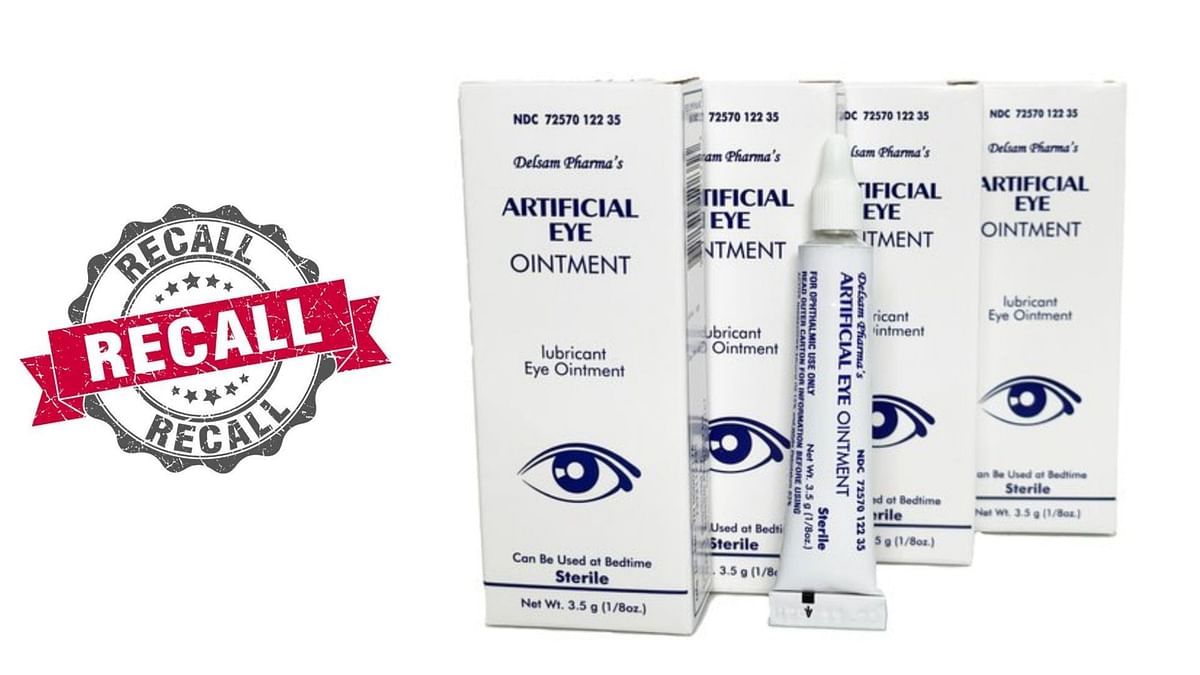
Contact an attorney if injured by recalled eye solution. Several eyedrop manufacturers have issued a recall of eyedrops and eyecare products in 2025 — here are the products that have been recalled. Millions of cartons of eye drops and other eye products have been recalled nationwide due to manufacturing and quality issues.


:quality(80)/cloudfront-us-east-1.images.arcpublishing.com/semana/OKQNZHBHFVEF3ALWFQXNT2GNO4.jpeg)





:max_bytes(150000):strip_icc()/Health-EyeOintmentRecall-d2f2b1fd023545a6b2081e48b395c42c.jpg)

Several Eyedrop Manufacturers Have Issued A Recall Of Eyedrops And Eyecare Products In 2025 — Here Are The Products That Have Been Recalled.
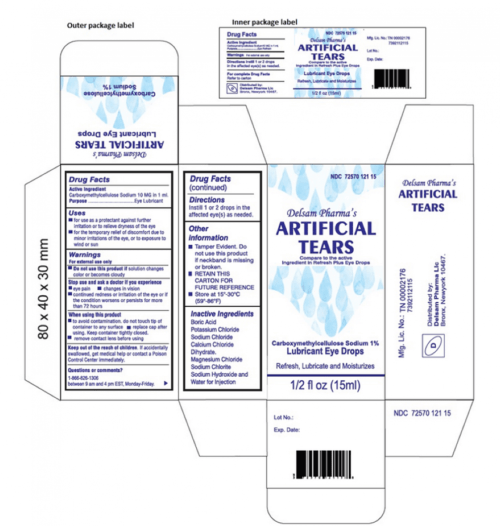
List of recalled eye care products the recall covers five different ophthalmic solutions distributed nationwide between may. Here are the eye care product recall list issued by the fda. The drops, used to relieve itchy, dry eyes, were also recalled due to a “lack of assurance of sterility,” according to the fda.
The Fda Recalled Eye Drops, Which Were Intended To Soothe Dry, Itchy Eyes, Because Of A “Lack Of Assurance Of Sterility.”
Here is what you need to know. The pharmaceutical lab brs analytical service, llc has issued a voluntary recall of. The products included in the recall were shipped between may 26, 2023, and april 21, 2025.
If You’re A Frequent Eye Drop User, Now’s The Time To Check Your Medicine Cabinet:
Contact an attorney if injured by recalled eye solution. Millions of cartons of eye drops and other eye products have been recalled nationwide due to manufacturing and quality issues.
